Bnt111 Clinical Trial
BNT111 is developed for the treatment of advanced melanoma in patients with metastatic tumors and as an adjuvant treatment after tumor resection. In addition efficacy analysis of the Lipo-MERIT study in a subset of 42 metastatic melanoma patients previously treated with a checkpoint-inhibitor CPI.
This Phase 2 clinical trial is based on previous results from the Phase 1 Lipo-MERIT dose escalation trial NCT02410733 that demonstrated a favorable safety profile in.
Bnt111 clinical trial
. Efficacy analysis in a subset of 42 checkpoint-inhibitor CPI-experienced metastatic melanoma patients shows that BNT111 mediates durable responses both as a single agent and in combination with anti-PD-1 antibodies. Interventional Clinical Trial Estimated Enrollment. This Phase 2 clinical trial is based on previous results from the Phase 1 Lipo-MERIT dose escalation trial NCT02410733 that demonstrated a favorable safety profile in 89 patients with advanced melanoma. This is an open-label randomized multi-site Phase II interventional trial designed to evaluate the efficacy tolerability and safety of BNT111 cemiplimab in anti-PD1-refractoryrelapsed patients with unresectable Stage III or IV melanoma.Alongside BNT111 BNT112 an experimental treatment against Castration Resistant Prostate Cancer currently in Phase 1 trials and BNT113 another mRNA treatment against HPV-positive head and neck. This Phase 2 clinical trial is based on previous results from the Phase 1 Lipo-MERIT dose escalation trial NCT02410733 that demonstrated a favorable safety profile in 89 patients with advanced melanoma. BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma Provided by GlobeNewswire Jun 18 2021 900 AM UTC. Treating patients who have cancer with vaccines that stimulate a targeted immune response is conceptually appealing but cancer vaccine trials have not been successful in late-stage patients with treatment-refractory tumours 12We are testing melanoma FixVac BNT111-an intravenously administered liposomal RNA RNA-LPX vaccine which targets four non-mutated tumour-associated.
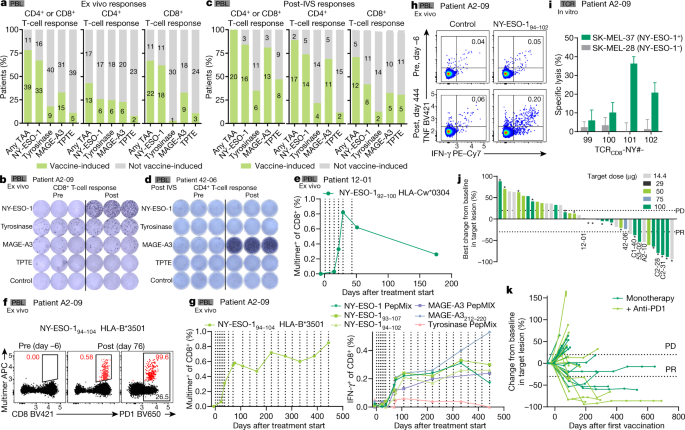
MAINZ Germany June 18 2021 GLOBE NEWSWIRE BioNTech SE NASDAQBNTX BioNTech or the Company announced today that the first patient has been treated in its BNT111 Phase 2 cancer. BioNTech also plans to start randomised Phase II trials with mRNA vaccine product candidates in two additional programs in 2021. None Open Label Primary Purpose. Durable objective responses by BNT111 were associated with activation and strong expansion of tumor-antigen-specific CD4 and CD8 T cells.
It is designed to elicit an immune response to four melanoma-associated antigens. BNT111 is one of the most advanced of five clinical-stage FixVac product candidates within BioNTechs development pipeline. We are currently studying BNT111 in an ongoing Phase 1 clinical trial. A phase 1 clinical trial demonstrated a favorable safety profile in 89 patients with advanced melanoma.
The contributions of BNT111 and cemiplimab will be delineated in single agent calibrator arms. These results were published in Nature in July 2020. BNT111 is one of the most advanced of five clinical-stage FixVac product candidates within BioNTechs development pipeline. BNT111 is currently conducting clinical trials.
First program from BioNTechs fully-owned mRNA cancer vaccine platform. --BioNTech SE announced today the publication of interim Phase 1 data for the Company s FixVac cancer vaccine program BNT111 in the journal Nature. BioNTech Publishes Data from mRNA-based BNT111 FixVac Melanoma Trial in Nature. Jun 18 2021 500AM EDT.
BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma Published. BNT111 is composed of four melanoma antigens NY-ESO-1 MAGE-A3 tyrosinase and TPTE and is the most advanced of five clinical-stage FixVac product candidates within. Phase 12a First-in-human Open-label Dose-escalation Trial With Expansion Cohorts to Evaluate Safety Pharmacokinetics. BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma.
MAINZ Germany July 30 2020 GLOBE NEWSWIRE. First program from BioNTechs fully-owned mRNA cancer vaccine platform FixVac treats patients in a randomized clinical Phase 2 clinical trial Phase 2 trial is based on positive results from Phase 1 Lipo-MERIT trial that demonstrated a favorable safety profile for BNT111 as well as durable objective responses observed in patients with melanoma who had progressed following prior. BNT111 Melanoma Vaccine Clinical Trials.
Biontech Se 2020 Foreign Issuer Report 6 K
Biontech Se 2020 Foreign Issuer Report 6 K
An Rna Vaccine Drives Immunity In Checkpoint Inhibitor Treated Melanoma Nature
Biontech Se 2020 Foreign Issuer Report 6 K
Cancer Testis Antigens From Serology To Mrna Cancer Vaccine Sciencedirect
Http Www Europarl Europa Eu Cmsdata 227701 Speaker 1 Andreas Kuhn Pdf
Biontech Se 2020 Foreign Issuer Report 6 K
Vaccines Free Full Text Evolution Of Cancer Vaccines Challenges Achievements And Future Directions Html
Https Investors Biontech De Static Files 37a925e5 D748 4c09 8e72 Fb5791cfa7a1
Biontech Se 2020 Foreign Issuer Report 6 K
The Limitless Future Of Rna Therapeutics Abstract Europe Pmc
Biontech Se 2020 Foreign Issuer Report 6 K
An Rna Vaccine Drives Immunity In Checkpoint Inhibitor Treated Melanoma Nature
Frontiers The Limitless Future Of Rna Therapeutics Bioengineering And Biotechnology
Clinical Trials Of Cancer Immunotherapy Involving Nanomedicines And Download Scientific Diagram
Efficacy analysis in a subset of 42 checkpoint-inhibitor CPI-experienced metastatic melanoma patients shows that BNT111 mediates durable responses both as a single agent and in combination with anti-PD-1 antibodies. We are currently studying BNT111 in an ongoing Phase 1 clinical trial.
Frontiers The Limitless Future Of Rna Therapeutics Bioengineering And Biotechnology
Phase 12a First-in-human Open-label Dose-escalation Trial With Expansion Cohorts to Evaluate Safety Pharmacokinetics.

Bnt111 clinical trial
. The contributions of BNT111 and cemiplimab will be delineated in single agent calibrator arms. Jun 18 2021 500AM EDT. MAINZ Germany July 30 2020 GLOBE NEWSWIRE. Treating patients who have cancer with vaccines that stimulate a targeted immune response is conceptually appealing but cancer vaccine trials have not been successful in late-stage patients with treatment-refractory tumours 12We are testing melanoma FixVac BNT111-an intravenously administered liposomal RNA RNA-LPX vaccine which targets four non-mutated tumour-associated.These results were published in Nature in July 2020. A phase 1 clinical trial demonstrated a favorable safety profile in 89 patients with advanced melanoma. BNT111 is one of the most advanced of five clinical-stage FixVac product candidates within BioNTechs development pipeline. Alongside BNT111 BNT112 an experimental treatment against Castration Resistant Prostate Cancer currently in Phase 1 trials and BNT113 another mRNA treatment against HPV-positive head and neck.
BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma Provided by GlobeNewswire Jun 18 2021 900 AM UTC. BNT111 is composed of four melanoma antigens NY-ESO-1 MAGE-A3 tyrosinase and TPTE and is the most advanced of five clinical-stage FixVac product candidates within. It is designed to elicit an immune response to four melanoma-associated antigens. This Phase 2 clinical trial is based on previous results from the Phase 1 Lipo-MERIT dose escalation trial NCT02410733 that demonstrated a favorable safety profile in 89 patients with advanced melanoma.
BioNTech also plans to start randomised Phase II trials with mRNA vaccine product candidates in two additional programs in 2021. BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma. BNT111 is currently conducting clinical trials. BNT111 is one of the most advanced of five clinical-stage FixVac product candidates within BioNTechs development pipeline.
First program from BioNTechs fully-owned mRNA cancer vaccine platform FixVac treats patients in a randomized clinical Phase 2 clinical trial Phase 2 trial is based on positive results from Phase 1 Lipo-MERIT trial that demonstrated a favorable safety profile for BNT111 as well as durable objective responses observed in patients with melanoma who had progressed following prior. Durable objective responses by BNT111 were associated with activation and strong expansion of tumor-antigen-specific CD4 and CD8 T cells. BioNTech Publishes Data from mRNA-based BNT111 FixVac Melanoma Trial in Nature. Interventional Clinical Trial Estimated Enrollment.
First program from BioNTechs fully-owned mRNA cancer vaccine platform. This Phase 2 clinical trial is based on previous results from the Phase 1 Lipo-MERIT dose escalation trial NCT02410733 that demonstrated a favorable safety profile in 89 patients with advanced melanoma. BNT111 Melanoma Vaccine Clinical Trials. This is an open-label randomized multi-site Phase II interventional trial designed to evaluate the efficacy tolerability and safety of BNT111 cemiplimab in anti-PD1-refractoryrelapsed patients with unresectable Stage III or IV melanoma.
BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma Published. None Open Label Primary Purpose. MAINZ Germany June 18 2021 GLOBE NEWSWIRE BioNTech SE NASDAQBNTX BioNTech or the Company announced today that the first patient has been treated in its BNT111 Phase 2 cancer. --BioNTech SE announced today the publication of interim Phase 1 data for the Company s FixVac cancer vaccine program BNT111 in the journal Nature.
Https Investors Biontech De Static Files 37a925e5 D748 4c09 8e72 Fb5791cfa7a1
An Rna Vaccine Drives Immunity In Checkpoint Inhibitor Treated Melanoma Nature
Biontech Se 2020 Foreign Issuer Report 6 K
An Rna Vaccine Drives Immunity In Checkpoint Inhibitor Treated Melanoma Nature
Clinical Trials Of Cancer Immunotherapy Involving Nanomedicines And Download Scientific Diagram
Http Www Europarl Europa Eu Cmsdata 227701 Speaker 1 Andreas Kuhn Pdf
Vaccines Free Full Text Evolution Of Cancer Vaccines Challenges Achievements And Future Directions Html
The Limitless Future Of Rna Therapeutics Abstract Europe Pmc
Biontech Se 2020 Foreign Issuer Report 6 K
Biontech Se 2020 Foreign Issuer Report 6 K
Biontech Se 2020 Foreign Issuer Report 6 K
Biontech Se 2020 Foreign Issuer Report 6 K
Cancer Testis Antigens From Serology To Mrna Cancer Vaccine Sciencedirect


Posting Komentar untuk "Bnt111 Clinical Trial"